The Peculiar Evolution of a Pair of Homologues on the Sex Chromosomes
The Y-located TSPY proto-oncogene has a X-located homologue, termed TSPX, which turns out to be a tumor suppressor on the X chromosome. TSPY and TSPX originated from the same ancestral gene but diverged in gene structure and functions during the evolution of the modern-day human sex chromosomes. TSPY had specialized in male-specific functions in spermatogenesis while TSPX maintained the functions of the ancestral gene. Both proteins harbor a conserved domain, termed SET/NAP domain, initially identified in the proto-oncogene SET and the nucleosome assembly proteins (NAP). They are key members of the SET/NAP family of proteins, which serve a variety of cellular functions, including cell proliferation, transcriptional regulation, histone modification and chromatin structure. TSPY and TSPX maintain similar genomic organization, with a unique exon 1, and conserved exon 2-5 coding for the conserved the SET/NAP domain (Figure 1). TSPX contains exon 6 and 7 encoding for an acidic domain, which is absent in TSPY. TSPY is one of the four genes on the male-specific region on the Y chromosome (MSY), which had diverged significantly from the respective homologues on the X chromosome and serves specialized male-specific functions. We surmise that the apparent truncation of the acidic domain from the ancestral gene during the evolution of TSPY could likely be related to its specialization in male-specific functions, which include spermatogonial/germ stem cell proliferation and meiotic division.
Based on the atomic structure deduced from a SET protein with truncation of its small acidic domain, we postulate that TSPY likely forms a dimer at its N-terminal helix with protruding SET/NAP domains, resembling a pair of earmuffs. TSPX, on the other hand, assume similar structure, but with an additional acidic tail extending from each of the earmuffs (Figure 1). Such differences in the protein structures between TSPY and TSPX could be significant in their differential actions in various biological functions, as discussed below.
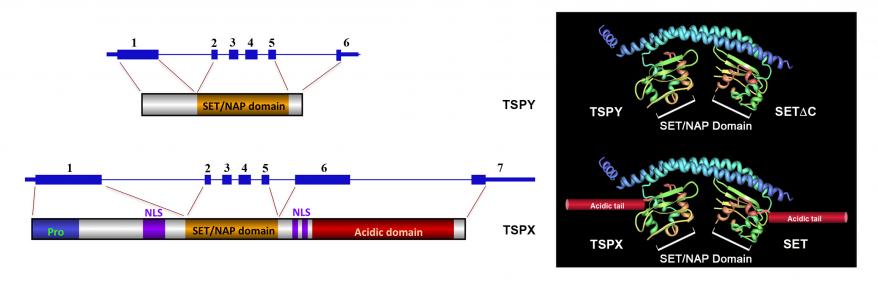
Figure 1. Gene organization, encoded proteins and likely atomic structures of TSPY, top, and TSPX, bottom.
References:
Lau Y-FC, Kido T and Li YM (2007). The TSPY Gene Family. In Y Chromosome and Male Germ Cell Biology (Y-FC Lau and WY Chan, Editors). Pp73-90, World Scientific Press, New Jersey.
Lau Y-FC, Li YM and Kido. Battle of the Sexes: Contrasting Roles of TSPY and TSPX in Human Oncogenesis, Asian J. Andrology, 2019 May-Jun; 21(3):260-269.PMID:29974883. PMCID: PMC6498724.
TSPY and TSPX Possess Contrasting Properties in Cell Cycle Regulation
TSPY is a repetitive gene on the Y chromosome. It is expressed in embryonic and adult testis and serves vital functions in germ stem cell proliferation and meiotic division, likely through its interaction and stimulation of cyclin B-CDK1 kinase activities. Proper gene dosage and expression levels have been associated with male fertility. Ectopic and/or elevated TSPY expression in incompatible cells promotes cell proliferation and oncogenesis. TSPX (also known as TSPYL2) is a single-copy gene on the X chromosome. It possesses contrasting properties to those of TSPY. Similar to TSPY, it interacts and co-localizes with cyclin B1 at the mitotic spindles in metaphase cells but represses the cyclin B-CDK1 phosphorylation activities. Over-expression of TSPX retards cell cycle progression, particularly at G2/M phase. Hence, TSPX could serves a housekeeping function(s) in modulating the transition of this cell cycle stage and maintaining the integrity of the G2/M checkpoints. Accordingly, TSPX is a tumor suppressor in human oncogenesis. Notably, this is the only pair of homologues on the sex chromosomes that serve contrasting functions at the two extremes of the human oncogenic spectrum.
To explore the molecular basis for their differential functions, we had conducted a series of domain mapping studies designed to identify the critical domain(s) important for their respective actions on the cyclin B-CDK1 phosphorylation. We showed that truncation of the carboxyl acidic domain renders TSPX to be stimulatory on cyclin B-CDK1 activities as TSPY. Significantly, transposition of this acidic domain to the carboxyl terminus of TSPY results in an inhibitory hybrid protein as TSPX. Hence, we identified the acidic domain of TSPX to be the inhibitory domain, responsible for its repression on cyclin B-CDK1 kinase activities.
Reference: Li Y, Lau YF. TSPY and its X-encoded homologue interact with cyclin B but exert contrasting functions on cyclin-dependent kinase 1 activities. Oncogene. 2008 Oct 16; 27(47):6141-50. PMID: 18591933
Differential Actions of TSPY and TSPX on Androgen Receptor Signaling
TSPY forms a positive feedback loop with androgen receptor (AR) and its constitutively active variants, such as AR-V7, in which TSPY expression is stimulated by AR/AR-V7 and the TSPY protein, in turn, binds to AR/AR-V7 and amplifies their transactivation of target genes, including its own gene. The interactive domains have been mapped to the SET/NAP domain of TSPY and the N-terminal and DNA-binding domain of AR/AR-V7. TSPX also binds AR/AR-V7 via its SET/NAP domain, but it represses the transactivation activities of AR/AR-V7 on their responsive genes, including its Y-homologue TSPY (Figure 2). Importantly, truncation of the carboxyl acidic domain in TSPX results in a stimulatory factor, exacerbating the transactivation of AR/AR-V7 on their target genes. Further, transposition of such acidic domain to the carboxyl terminus of TSPY renders the hybrid protein to be inhibitory in AR/AR-V7 transcriptional activities. Since TSPY is a target gene for AR/AR-V7, such inhibitory actions suggest that the X-located TSPX could regulate the expression of its Y-homologue TSPY in various biological and disease processes, involving male sex hormone and its receptors.
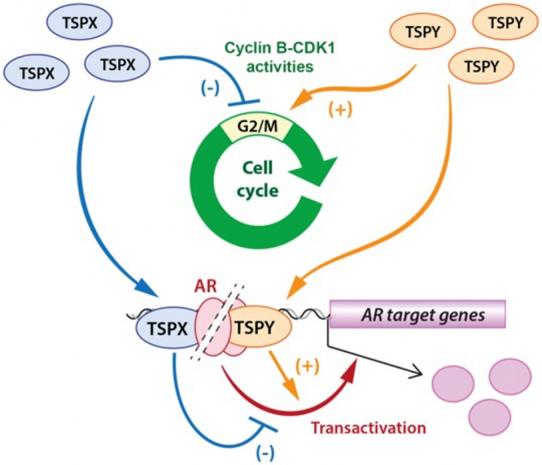
Figure 2. TSPY and TSPX possess contrasting actions on cyclin B-CDK1 phosphorylation activities and differentially regulate the cell cycle progression at the G2/M stage (top); and exert differential actions on androgen receptor (AR) transactivation of responsive genes (bottom). Both opposing functions could be attributed to the carboxyl acidic domain in TSPX, absent in TSPY.
Reference: Li Y, Zhang DJ, Qiu Y, Kido T, Lau YC. The Y-located proto-oncogene TSPY exacerbates and its X-homologue TSPX inhibits transactivation functions of androgen receptor and its constitutively active variants. Hum Mol Genet. 2017 03 01; 26(5):901-912. PMID: 28169398. PMCID: PMC6075507
Transcriptome analysis of the androgen responsive prostate cancer cell line, LNCaP, showed that TSPY amplifies while TSPX represses the expression of the endogenous AR target genes as well as a significant number of common canonical pathways in growth and oncogenesis. Again, domain mapping studies demonstrated that the carboxyl acidic domain is important for TSPX downregulation of cancer drivers/oncogenes, including MYC and MYB in prostate cancer cells. These results support the role of TSPY as a male-specific oncogene and TSPX as a X-located tumor suppressor in the hormone-responsive prostate cancer.
Reference: Kido T, Li YM, Tanaka Y, Dahiya R, Lau YC. The X-linked tumor suppressor TSPX downregulates cancer-drivers/oncogenes in prostate cancer in a C-terminal acidic domain dependent manner. Oncotarget, 2019, Feb 19, 10(15):1491-1505. PMID:30863497. PMCID:PMC6407674
TSPY and TSPX in Liver Cancer
Liver cancer, particularly hepatocellular carcinoma (HCC), shows significant male biases in incidence, metastatic progression and poor treatment responses. Currently, the exact etiologies for such sex differences are uncertain. Male sex hormone and AR have been postulated to play key roles in both viral and non-viral hepatocarcinogenesis, thereby exerting male influences in the disease processes. The sex chromosomes, in particular TSPY and TSPX, could potentially exert various influences in the HCC pathogenesis. As a Y-located proto-oncogene, its aberrant expression in hepatocytes could exacerbate cell proliferation and androgen/AR transactivation of oncogenic genes and pathways. On the other hand, as a X-located tumor suppressor, mutation/inactivation of TSPX could render males without its tumor suppressing functions since males have only one X chromosome. Indeed, TSPY positive patients have poorer survival than the TSPY negative/female patients. Patients with high TSPX expression tend to have better survival and outcomes in HCC. Significantly, recent emphasis on translational applications of anti-androgen/AR therapeutics on HCC have highlighted the need for research on TSPY/TSPX and AR/AR-V7 actions in liver cancer biology and therapeutic strategies.
References:
Kido T, Lo RC, Li Y, Lee J, Tabatabai ZL, Ng IO, Lau YF. The potential contributions of a Y-located protooncogene and its X homologue in sexual dimorphisms in hepatocellular carcinoma. Hum Pathol. 2014 Sep; 45(9):1847-58. PMID: 25017435
Kido T, Lau YC. Identification of a TSPY co-expression network associated with DNA hypomethylation and tumor gene expression in somatic cancers. J Genet Genomics. 2016 Oct 20; 43(10):577-585. PMID: 27771326
In addition to cell proliferation and male sex hormone/receptor functions, TSPY and TSPX also show differential actions in viral hepatocarcinogenesis, particularly on hepatitis B virus (HBV). HBV replication is regulated by androgen via AR transactivation of various viral components, including the viral oncogene, HBx, which has been postulated to play critical role in HBV-mediated carcinogenesis. We showed that TSPX interacts simultaneously with HBx protein and RPN3, a subunit of the 19S component of proteasome via its carboxyl acidic domain. Such interactions result in abolishment of the protective function of RPN3 on HBx, thereby promoting a proteasomal degradation of HBx. TSPY, on the other hand, does not harbor an acidic domain, and hence has no effects on HBx stability.
Reference: Kido T, Ou JH, Lau YF. The X-linked tumor suppressor TSPX interacts and promotes degradation of the hepatitis B viral protein HBx via the proteasome pathway. PLoS One. 2011; 6(7):e22979. PMID: 21829568. PMCID: PMC3146538
Transcriptome analysis of high and low/no TSPY-expressing HCC specimens showed that TSPY upregulates directly or indirectly various genes associated with cell-cycle and cell-viability and suppresses cell-death related genes. Such findings could be correlated with those between HCC HuH-7 cells with and without TSPY over-expression. Similar to other cell types, over-expression of TSPY accelerates cell proliferation in HuH-7 cells. Further comparative analysis identified key TSPY-downstream genes involved in cell cycle events, particularly during mitosis (Figure 3). Upregulation of these mitotic genes could potentially be associated with the TSPY exacerbation of cyclin B-CDK1 phosphorylation, a key regulatory event during mitosis.
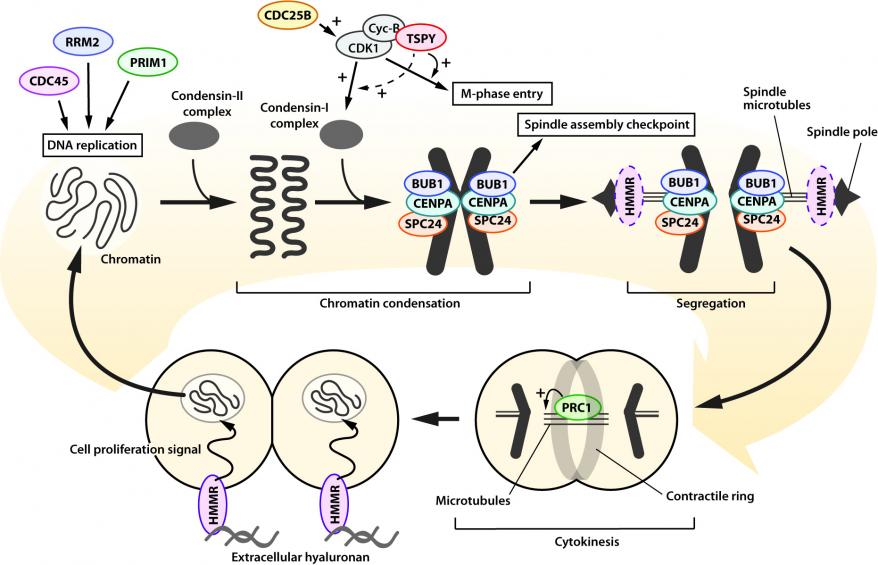
Figure 3. Schematic diagram illustrating the functions of TSPY and TSPY-downstream genes in cell cycle events, particularly mitosis, in HCC. From top-left; CDC45, RRM2 and PRIM1 participate in DNA replication at S-phase; CDC25B activates cyclin-B/CDK1 complex and promotes M-phase entry. TSPY binds cyclin-B/CDK1 complex and enhances its activity. BUB1, CENPA, and SPC24 interact with the condensed chromatin and participate in spindle assembly checkpoint and chromatin segregation respectively. HMMR promotes spindle pole formation; PRC1 binds microtubules and modulates cytokinesis. HMMR also activates the signaling cascades of cell-proliferation via extracellular hyaluronan.
Reference: Kido T, Lau YC. The Y-linked proto-oncogene TSPY contributes to poor prognosis of the male hepatocellular carcinoma patients by promoting the pro-oncogenic and suppressing the anti-oncogenic gene expression. Cell & Bioscience 2019, 9:22. PMID: 30867900. PMCID: PMC6399826