TSPY as a Proto-Oncogene on the Human Y Chromosome
The Y chromosome is critical for male development and physiology. The involvement of the Y chromosome in human cancers has been highlighted by the gonadoblastoma locus (GBY), initially proposed to explain the significantly high incidence of gonadoblastoma, a benign tumor cell tumor, among patients of disorders of sex development (DSD) harboring residual Y chromosome sequences. The GBY gene(s) is postulated to serve a normal function(s) in the testis, but exert oncogenic activities in dysfunctional gonadal environments, such as streaked dysgenetic gonads. Numerous studies, by our laboratory and others, suggest that the testis-specific protein Y-encoded (TSPY) gene is the putative gene for GBY. Indeed, TSPY is abundantly expressed in gonadoblastoma, testicular germ cell tumors (TGCTs), seminomas, selected nonseminomas, intracranial germ cell tumors of male origin, and somatic cancers including prostate cancer, hepatocellular carcinoma (HCC), head and neck cancer, lung cancer and melanoma. High levels of TSPY expression in HCC is associated with poor survival of the patients.
TSPY is tandemly repeated 20-60 times and constitutes the largest block (up to 1 MB of DNA) of functional gene array in the human genome. Variations of its gene-copy number have been observed among the normal populations, and could be associated with male fertility. TSPY gene array is a hot spot for genetic variations on the human Y chromosome. We demonstrated that in a humanized mouse strain, TgTSPY9, in which the human TSPY is tandemly integrated on the mouse Y chromosome, the TSPY transgene is aberrantly activated in the LADY mouse model of prostate cancer, suggesting its genetic/epigenetic instability during oncogenesis.
References:
Lau YF. Gonadoblastoma, testicular and prostate cancers, and the TSPY gene. Am J Hum Genet. 1999 Apr; 64(4):921-7. PMID: 10090875. PMCID: PMC1377814
Li Y, Vilain E, Conte F, Rajpert-De Meyts E, Lau YF. Testis-specific protein Y-encoded gene is expressed in early and late stages of gonadoblastoma and testicular carcinoma in situ. Urol Oncol. 2007 Mar-Apr; 25(2):141-6. PMID: 17349529
Kido T, Schubert S, Hatakeyama S, Ohyama C, Schmidtke J, Lau YF. Expression of a Y-located human proto-oncogene TSPY in a transgenic mouse model of prostate cancer. Cell Biosci. 2014; 4(1):9. PMID: 24528896. PMCID: PMC3942074
Over-expression of TSPY accelerates cell proliferation, promotes tumorigenicity in mice, up-regulates oncogenic genes and pathways, and represses tumor suppressors. Transgenic mouse modeling demonstrated that TSPY is capable of expressing in female germ cells and promotes gonadoblastoma-like structure in female transgenic mice, suggesting that TSPY is a Y-located proto-oncogene exerting cell proliferative properties and oncogenic functions when inappropriately expressed in dysfunctional germ cells and/or somatic cells incapable of entering male meiosis.
References:
Oram SW, Liu XX, Lee TL, Chan WY, Lau YF. TSPY potentiates cell proliferation and tumorigenesis by promoting cell cycle progression in HeLa and NIH3T3 cells. BMC Cancer. 2006; 6:154. PMID: 16762081. PMCID: PMC1526451
Kido T, Schubert S, Schmidtke J, Lau YFC. Expression of the human TSPY gene in the brains of transgenic mice suggests a potential role of this Y chromosome gene in neural functions. J Genet Genomics. 2011 May 20; 38(5):181-91. PMID: 21621739
TSPY and cancer stem cells. TSPY is expressed at high levels in the premalignant precursor, carcinoma-in-situ(CIS)/germ cell neoplasia in situ (GCNIS), gonadoblastoma and testicular germ cell tumors. Its expression is closely correlated with cancer stem cell marker, i.e. CD33, and specific germ cell tumor markers, i.e. placental alkaline phosphatase, c-Kit and Oct4. Since CIS/GCNIS has been considered to be a cancer stem cell for gonadoblastoma and all testicular germ cell tumors, TSPY could potentially be important for either the maintenance or oncogenic differentiation of CIS/GCNIS in germ cell tumorigenesis.
References
Li Y, Tabatabai ZL, Lee TL, Hatakeyama S, Ohyama C, Chan WY, Looijenga LH, Lau YF. The Y-encoded TSPY protein: a significant marker potentially plays a role in the pathogenesis of testicular germ cell tumors. Hum Pathol. 2007 Oct; 38(10):1470-81. PMID: 17521702. PMCID: PMC2744854
Lau Y-FC, Li YM and Kido. Battle of the Sexes: Contrasting Roles of TSPY and TSPX in Human Oncogenesis, Asian J. Andrology, 2019 May-Jun; 21(3):260-269.PMID:29974883. PMCID: PMC6498724.
Functions of TSPY Gene in Normal Physiology and Diseases
Our studies suggest that TSPY serves normal functions in male stem germ cell propagation and meiotic division. It is expressed in gonocytes of embryonic testis and co-expressed with Oct4 and c-Kit in spermatogonia and spermatocytes of the adult testis. It interacts with the type B cyclins and stimulates the cyclin B-CDK1 phosphorylation activities, potentially critical for spermatogonial renewal and male meiosis. TSPY and cyclin B1 are co-expressed tightly during spermatocyte differentiation. We hypothesize that TSPY stimulation of cyclin B-CDK1 phosphorylation could be important for the two rounds of meiotic divisions without an intermediate interphase during spermatogenesis, a unique feature for male meiosis.
TSPY co-localizes with cyclin B1 during interphase and preferentially at the spindles at various stages of mitosis of somatic cells (Figure 1). TSPY stimulates the cyclin B-CDK1 phosphorylation activities and promotes a rapid transition of the G2/M phase. Aberrant and high-level expressions of TSPY in incompatible cells, such as dysfunctional germ cells and somatic cells incapable of entering male meiosis, stimulates cell proliferation, disrupts the G2/M checkpoints in the cell cycle, and potentially induces genome instability, thereby promoting oncogenesis of the affected cells. Hence, TSPY is a male-specific proto-oncogene on the Y chromosome, contributing to the oncogenesis in male-specific/related cancers, such as gonadoblastoma, testicular germ cell tumors and prostate cancer, and male biases in somatic cancers, such as hepatocellular carcinoma, head and neck and lung cancers.

Figure 1. Co-localization of TSPY and Cyclin B1 during mitosis of somatic cells. A. TSPY distributions during various mitotic phases. B. TSPY and C. Cyclin B1 are preferentially localized in the mitotic spindles in metaphase cells, D. Co-localization of TSPY and Cyclin B1 in a metaphase cell.
References:
Li Y, Lau YF. TSPY and its X-encoded homologue interact with cyclin B but exert contrasting functions on cyclin-dependent kinase 1 activities. Oncogene. 2008 Oct 16; 27(47):6141-50. PMID: 18591933
Lau YF, Li Y, Kido T. Gonadoblastoma locus and the TSPY gene on the human Y chromosome. Birth Defects Res C Embryo Today. 2009 Mar; 87(1):114-22. PMID: 19306348
Lau YF, Li Y, Kido T. Role of the Y-located putative gonadoblastoma gene in human spermatogenesis. Syst Biol Reprod Med. 2011 Feb; 57(1-2):27-34. PMID: 21204751
TSPY Forms a Positive Feedback Loop with Androgen Receptor
As a proto-oncogene on the Y chromosome, TSPY plays key roles in male-specific/biased cancers. Further, its expression is regulated by the male sex hormone androgen via binding of the androgen receptor (AR) on its promoter. Significantly, we further demonstrated that TSPY physically interacts with AR and its constitutively active variants, such as AR-V7, and stimulates the AR and AR-V7 transactivation of responsive genes in ligand dependent and independent manners respectively. These findings suggest the existence of a positive feedback loop between the TSPY and AR/AR-V7, in which TSPY is transcriptionally up-regulated by AR/AR-V7 and its protein, in turn, binds to AR/AR-V7 and exacerbates the AR/AR-V7 transactivation of its own gene as well as other AR/AR-V7 responsive targets (Figure 2). Accordingly, a Y-located oncogene amplifies its own expression through exacerbating the transcriptional activities of the male sex hormone receptors, and promotes oncogenesis in specific cancers, such as prostate cancer, in which the AR and AR variants play key roles in the initiation and progression to metastatic castration resistant prostate cancer (CRPC). Indeed, TSPY expression has been detected in various stages of prostate cancer and is associated with poor prognosis of the patients. Further, TSPY expression has also been detected in liver cancer, head and neck cancer, lung cancer and melanoma, some of which also show male biases in the incidence and/or pathogenesis and/or are subjected to sex hormone/receptor modulations. Hence, the TSPY-AR/AR-V7 positive feedback loop could play key roles in the oncogenic processes of these male-biased cancers.
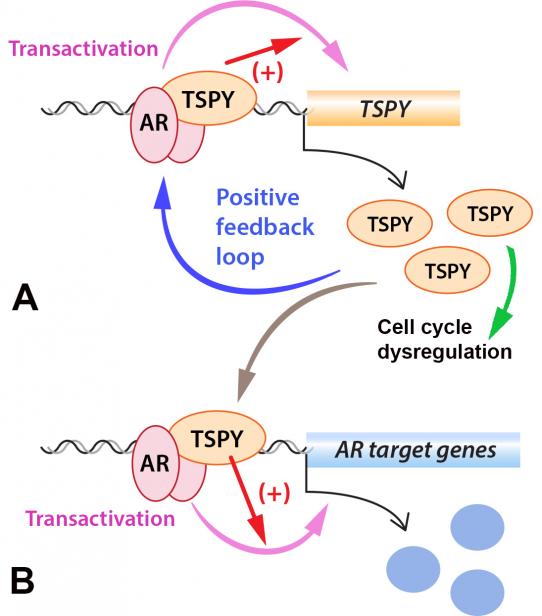
Figure 2. A positive feedback loop between TSPY and AR/AR-V7, in which AR/AR-V7 bind to the TSPY promoter and stimulate its expression in a ligand-dependent and independent manners, the TSPY protein, in turn, interacts and exacerbates AR/AR-V7 transactivation of its own gene (A) as well as AR/AR-V7 target genes (B).
Reference: Li Y, Zhang DJ, Qiu Y, Kido T, Lau YC. The Y-located proto-oncogene TSPY exacerbates and its X-homologue TSPX inhibits transactivation functions of androgen receptor and its constitutively active variants. Hum Mol Genet. 2017 03 01; 26(5):901-912. PMID: 28169398. PMCID: PMC6075507